Electron Spectroscopy for Chemical Analysis (ESCA)
advertisement

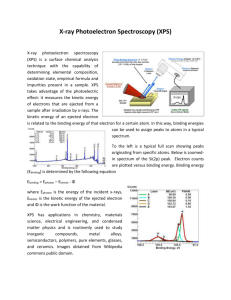
125: 583 Biointerfacial Characterization Electron Spectroscopy for Chemical Analysis (ESCA) Professor Theodore Madey Case Study Discussant: Prabhas Moghe Outline of Lecture • Introduction • Principles of ESCA – The photoelectron effect – Instrumentation -- how measurements are made • Analysis Capabilities – Elemental analysis – Chemical state analysis (core level shifts) – More complex effects • • • • Surface Sensitivity Applications Comparisons with other techniques Discussion of Journal Paper (Biointerfacial Case Study) Motivation • Why is surface analysis important for Biomaterials and Biological Systems? • Interactions between solid surfaces and biological systems --- Biocompatibility --- Biomolecular separations --- Cell culture --- Marine Fouling --- Biosensors 1. Introduction -- ESCA provides unique information about chemical composition And chemical state of a surface -- useful for biomaterials -- advantages -- surface sensitive (top few monolayers) -- wide range of solids -- relatively non-destructive -- disadvantages -- expensive, slow, poor spatial resolution, requires high vacuum 2. Principles of ESCA • ESCA (also known as X-ray photoelectron spectroscopy, XPS) is based on the photoelectron effect. A high energy X-ray photon can ionize an atom, producing an ejected free electron with kinetic energy KE: KE h BE • h =photon energy (e.g., for Al K , h 1486.6 eV) BE=energy necessary to remove a specific electron from an atom. BE orbital energy Basics of Light, EM Spectrum, and X-rays Light can take on many forms. Radio waves, microwaves, infrared, visible, ultraviolet, X-ray and gamma radiation are all different forms of light. The energy of the photon tells what kind of light it is. Radio waves are composed of low energy photons. Optical photons--the only photons perceived by the human eye--are a million times more energetic than the typical radio photon. The energies of X-ray photons range from hundreds to thousands of times higher than that of optical photons. Very low temperatures (hundreds of degrees below zero Celsius) produce low energy radio and microwave photons, whereas cool bodies like ours (about 30 degrees Celsius) produce infrared radiation. Very high temperatures (millions of degrees Celsius) produce Xrays. • • • • All energies expressed in electron volts (eV); 1 eV=1.6x10-19 J In ESCA, you know h & you measure KE; this determines BE. Photoelectron process: Consider an ensemble of C atoms. Each C atom has 6 electrons in 1s, 2s, 2 p orbitals: C 1s2 2s2 2 p2 • Different orbitals give Different peaks in spectrum • Peak intensities depend on Photoionization cross section (largest for C 1s) • Extra peak: Auger emission Instrumentation: How are measurements made? • Essential components: • Sample: usually 1 cm2 • X-ray source: Al: 1486.6 eV; Mg 1256.6 eV • Electron Energy Analyzer: 100 mm radius concentric hemispherical analyzer; vary voltages to vary pass energy. • Detector: electron multiplier (channeltron) • Electronics, Computer • Note: All in ultrahigh vacuum (<10-8 Torr) (<10-11 atm) • State-of-the-art small spot ESCA: 10 mm spot size. 3. Analysis Capabilities 3d3/2,5/2 • Elemental Analysis: atoms have valence and core electrons: Core-level Binding energies provide unique signature of elements. • Quantitative analysis: measure intensities, use standards or tables of sensitivity factor Ag: Z=47 Be careful: elements with similar BEs C1s & Ru3d; Ar2p & Rb 3p Chemical State Identification C1s – 4 peaks! Core level chemical shifts: For the same atom in two different chemical states: BE BE(2) BE(1) KE(1) KE(2) Explanation of chemical shifts r If a charge q is added to (or removed from) the valence shell due to chemical bond formation, the electrostatic potential felt by the electron inside the atom is changed. E ~ q/r ~ BE - (BE)o (Si2p BE increases) • When atom loses valence charge (Si0 --> Si4+ ) BE increases. • When atom gains valence charge (O --> O--) BE decreases. • Chemical shift of C1s Also: • final state effects • more complex effects -spin-orbit splitting -shake-up, shake-off -Auger electron emission Important factor is surface sensitivity; short mean free path l for Inelastic electron scattering. • 95% of signal comes from top layer (t=3l) e.g., 50 eV electrons, l~5Å, t < 15Å 1200 - eV electrons, l~20Å , t< 60Å Enhance surface sensitivity by grazing take-off. 5. Applications -- Surface contamination -- Failure analysis -- Effects of surface treatments -- Coating, films -- Tribological effects -- Depth Profiling (Ar+ sputtering) F1s C1s ESCA studies of polyimide Pyromellitic dianhydride -- oxydianiline • PMDA - ODA C KLL Auger Applications to biomaterials and biointerfaces • Biological interfaces have a limited number of elements (C, H, O, N, S, P, Si) • Extracting useful surface information is challenging. • ESCA can be used to (a) detect the presence of adsorbed proteins. (b) estimate the amount of protein present (c) resolution of one protein from another is difficult since many proteins share chemical features. When spectra are taken as a function of take-off angle, Useful information can be obtained, for example, for the uniformity of an overlayer; fraction covered; protein film thickness; and orientation of protein in the film. The table below is used to determine which surface analysis techniques would be most appropriate to solve problems in specific application areas. AES XPS TOF-SIMS Probe beam Analysis beam Electrons Electrons Photons Electrons Ions Ions Sampling Depth 5-50 Å 5-50 Å 1-10 Å Detection Limits 1 x 10-3 1 x 10-4 1 x 10-6 Information Elemental, SEM Elemental, Chemical Elemental, Chemical, Molecular Spatial Resolution ~100 Ao ~10 mm ~1000 Ao Restriction Inorganics Few Quantification Standards Required (e-beam damage of organics a major problem) Discussion of Journal Paper • • • Biomaterials 27 (2006) 691-701; Fabrication, characterization, and biological assessment of multilayered DNA-coatings for biomaterial purposes van den Beucken JJ, Vos MR, Thune PC, Hayakawa T, Fukushima T, Okahata Y, Walboomers XF, Sommerdijk NA, Nolte RJ, Jansen JA. Received 30 May 2005; accepted 21 June 2005. Available online 1 August 2005. • • Abstract This study describes the fabrication of two types of multilayered coatings onto titanium by electrostatic selfassembly (ESA), using deoxyribosenucleic acid (DNA) as the anionic polyelectrolyte and poly-d-lysine (PDL) or poly(allylamine hydrochloride) (PAH) as the cationic polyelectrolyte. Both coatings were characterized using UV-vis spectrophotometry, atomic force microscopy (AFM), X-ray photospectroscopy (XPS), contact angle measurements, Fourier transform infrared spectroscopy (FTIR), and for the amount of DNA immobilized. The mutagenicity of the constituents of the coatings was assessed. Titanium substrates with or without multilayered DNA-coatings were used in cell culture experiments to study cell proliferation, viability, and morphology. Results of UV-vis spectrophotometry, AFM, and contact angle measurements clearly indicated the progressive build-up of the multilayered coatings. Furthermore, AFM and XPS data showed a more uniform build-up and morphology of [PDL/DNA]-coatings compared to [PAH/DNA]coatings. DNA-immobilization into both coatings was linear, and approximated 3 μg/cm2 into each doublelayer. The surface morphology of both types of multilayered DNA-coatings showed elevations in the nanoscale range. No mutagenic effects of DNA, PDL, or PAH were detected, and cell viability and morphology were not affected by the presence of either type of multilayered DNA-coating. Still, the results of the proliferation assay revealed an increased proliferation of primary rat dermal fibroblasts on both types of multilayered DNA-coatings compared to non-coated controls. The biocompatibility and functionalization of the coatings produced here, will be assessed in subsequent cell culture and animal-implantation studies. • Keywords: AFM; Cell culture; Cell morphology; Cell proliferation; Cell viability; Electrostatic self-assembly; Fibroblast; FTIR; MIT assay; Mutagenicity; Nanotopography; SEM; Surface modification; Titanium Experimental Procedures Polycationic polyelectrolytes PAH PDL The results PDL/DNA PAH/DNA Ti peaks seen No Ti Peaks Mg (?) Auger peaks – due to impurity counter ions?