Ch 2: A Mathematical Toolkit
advertisement
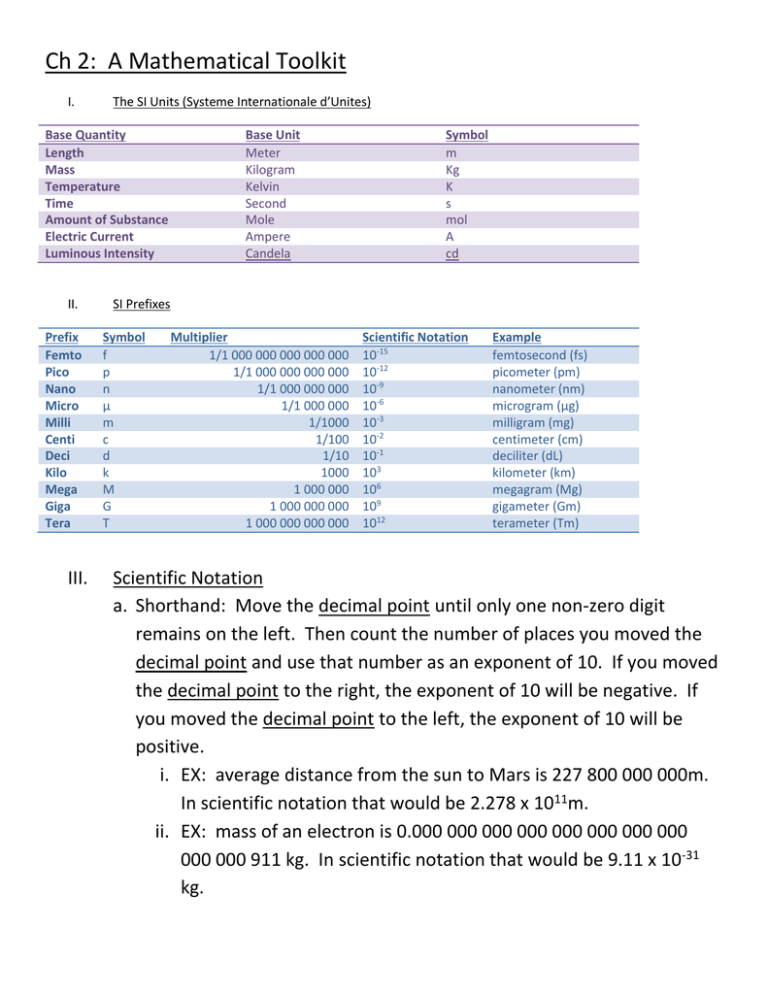
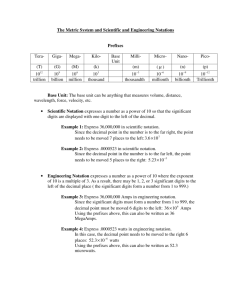
Ch 2: A Mathematical Toolkit I. The SI Units (Systeme Internationale d’Unites) Base Quantity Length Mass Temperature Time Amount of Substance Electric Current Luminous Intensity II. Prefix Femto Pico Nano Micro Milli Centi Deci Kilo Mega Giga Tera III. Base Unit Meter Kilogram Kelvin Second Mole Ampere Candela Symbol m Kg K s mol A cd SI Prefixes Symbol f p n µ m c d k M G T Multiplier 1/1 000 000 000 000 000 1/1 000 000 000 000 1/1 000 000 000 1/1 000 000 1/1000 1/100 1/10 1000 1 000 000 1 000 000 000 1 000 000 000 000 Scientific Notation 10-15 10-12 10-9 10-6 10-3 10-2 10-1 103 106 109 1012 Example femtosecond (fs) picometer (pm) nanometer (nm) microgram (µg) milligram (mg) centimeter (cm) deciliter (dL) kilometer (km) megagram (Mg) gigameter (Gm) terameter (Tm) Scientific Notation a. Shorthand: Move the decimal point until only one non-zero digit remains on the left. Then count the number of places you moved the decimal point and use that number as an exponent of 10. If you moved the decimal point to the right, the exponent of 10 will be negative. If you moved the decimal point to the left, the exponent of 10 will be positive. i. EX: average distance from the sun to Mars is 227 800 000 000m. In scientific notation that would be 2.278 x 1011m. ii. EX: mass of an electron is 0.000 000 000 000 000 000 000 000 000 000 911 kg. In scientific notation that would be 9.11 x 10-31 kg. IV. Converting Units: Factor-label Method a. An easy way to convert a quantity expressed in one unit to that quantity in another unit is to use a Conversion Factor, a relationship between two units. A conversion factor is a multiplier equal to 1. i. EX: Because 1 kg equals 1000g, you can construct the following conversion factors: 1 = 1kg / 1000g or 1 = 1000g/1kg ii. EX: Unit labels cancel just like algebraic quantities. This method of converting one unit to another is called the Factor-Label Method. 465g = (465g)(1kg / 1000g) = 465g x 1kg / 1000g = 0.465kg V. Significant Digits a. The valid digits in a measurement are called the Significant Digits. Rules for identifying Significant Digits: i. All non-zero digits are significant. ii. Zeros between two other significant digits are always significant. iii. All final zeros after the decimal point are significant. iv. Zeros used solely as placeholders are not significant b. HINT: Written in scientific notation, the significant digits of any number are equal to the number of digits multiplied by the power of 10. Practice Problems 1) Express the following quantities in scientific notation: a. 450 000 m b. 86 000 000 000 s c. 0.000 508 kg d. 0.0003600 kg 2) Convert each of the following length measurements as directed: a. 1.1 cm to meters b. 76.2 pm to millimeters c. 2.1 km to meters d. 2.278 x 104 m to kilometers 3) State the number of significant digits in each measurement: a. 2804 m b. 0.0029 m c. 4.6 x 105 m d. 75.00 m e. 1.20 x 10-4 m