What is GC/MS?
advertisement

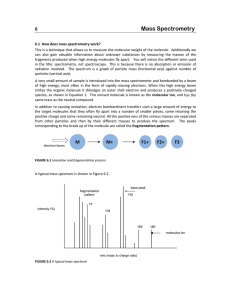
Interfacing Gas Chromatography with Mass Spectroscopy and Infra Red Spectroscopy Is this a large expensive detector…. Or a separation prior to analysis…. Early Use of Mass Spectroscopy • Quantitative methods for determination of the components in complex hydrocarbon mixtures • Later used for the identification and structural analysis of complex compounds • Method requires samples that are “clean” or interpretation is confusing Principles of measurements • As an identification method: – When a given molecular species is impacted with an electron beam, a family of positive particles are produced – The mass distribution of the particles are characteristic of the parent species Interfacing GC with Spectroscopic Methods - Early • eluates from column collected as separate fractions after being detected - composition measured by Mass Spectrometry or IR • Limitation - small (micromolar) composition of the solute • procedure still useful for qualitative analysis of multi-component Application of a Selective Detector - Modern • The detector monitors the column effluent continuously • Need computers to control instruments and store spectral data for display of spectrum and chromatograms Interfacing Gas Chromatography and Mass Spectroscopy (GC/MS) GC/ Mass Spectrometry • GC equipment can be directly interfaced with rapid-scan Mass Spectrometers • The flow rate is usually small enough to feed directly into the ionization chamber of the Mass Spectrometer • Packed columns use a jet separator, which removes the carrier gas for the analyte GC/ MS • Increase momentum of heavier analyte molecules so that 50% or more go into the skimmer • Lighter helium molecules are deflected by vacuum and pumped away • Use to identify components present in natural and biological systems – odor/flavor of foods - pollutants What is GC/MS? • Gas chromatography/mass spectrometry (GC/MS) is the synergistic combination of two powerful analytic techniques. • The gas chromatography separates the components of a mixture in time • The mass spectrometer provides information that aids in the structural identification of each component What is GC/MS? What is GC/MS? The GC/MS Interface • Transports the effluent from the gas chromatograph to the mass spectrometer • Analyte must not condense in the interface • Analyte may not decompose before entering the mass spectrometer ion source • The gas load entering the ion source must be within pumping capacity of the mass spectrometer GC/MS Interfaces • Capillary Columns • Macrobore and Packed Columns Capillary Columns • Insert exit end of column into ion source • Under normal operating conditions, the mass spectrometer can handle the entire effluent of the column • Must heat the capillary column to prevent condensation • Surface of columns must be inactive Macrobore and Packed Columns • Effluent must be reduced before entering ion source • Splitting the effluent results in a loss of sensitivity • Enrichment devices are used – Jet Separators are most common Jet Separator • Two capillary tubes aligned with a small space between them. (1 mm) • A vacuum is created between the two tubes using a rotary pump • The GC effluent enters the vacuum region, those molecules which continue in the same direction enter the second capillary tube and continue to the ion source Jet Separator • The carrier gas molecules are more easily diverted from the linear path by collisions • The analyte molecules are much larger and carry more momentum • The surface of the separator must be inactive and a reasonably even temperature • Prone to leaks Resolution and Mass Accuracy • With a modern mass spectrometer, it is possible to measure the mass of an ion to 1ppm with a resolution of 100,000 or better • GC/MS scanning conditions are limited to 5-10 ppm mass accuracy and resolution is only between 2,000 and 10,000. • These limitations are usually sufficient to allow for only a few reasonable and possible compositions Resolution and Mass Accuracy • Resolution can be increased by restricting the height and the width of the ion beam • A compromise must be made between minimizing mass interference and signal intensity for low levels of material • Gas chromatograph eliminates most compounds that cause mass interference. • Principle cause of peak overlap is the internal mass standard. Uses for GC/MS • May separate, analyze and identify unknown mixutres • May separate, and analyze known mixtures • For sample GC/MS experiments check out: – http://www.lehigh.edu/~ingcms/ingcms.html Complex Mass Spectrometer Detectors • Display modes - real time or computer reconstructed • Each has a choice of total ion current chromatogram or selected ion current chromatogram • Each can be generated on to a computer screen for print out Ion Trap Detector • compact - less expensive than quadropole • simplest mass detector for use in GC • ions are created form eluted sample by electron impact or chemical ionization • stored in radio-frequency field • ions injected from the storage area to a detector ITD • Ejection is controlled so the scanning of mass to charge ratio is possible Gas Chromatography Infrared Spectrometry (GC/IR) • • • • • Instrumentation/Interface Advantages Problems/Cons Solutions Practical Applications Infrared Spectrometry • Is especially useful for qualitative analysis of functional groups and other structural features • measuring concentrations is possible • establish identity of unknown compound with standard Instrumentation/Interface • Infrared Spectrophotometer determines the relative strengths and positions of the infrared region and plots the information on calibrated paper • Gas Chromatograph partitions the sample as it passes through the column • The two can be linked through glass column or vacuum tubes and other devices on more expensive equipment Fourier-Transform Infrared Spectroscopy (FTIR) Overcomes the problem of scanning for a collected sample or monitoring one wavelength Fourier Transform IR • Mechanically simple • Fast, sensitive, accurate • Internal calibration • No tracking errors or stray light FTIR • Analyze all wavelengths simultaneously • signal decoded to generate complete spectrum • can be done quickly • better resolution • more resolution • However, . . . Gas Chromatography / Infrared Spectrometry • Capillary GC with IR specs can enable the separation and identifying the compounds • The interface between the column and the detector is the main detail • Small pipe (length 10-40 cm, diameter 1-3 mm) connected to column by narrow tubing • Transmission of radiation occurs by multiple reflection off the wall GC/ IR • Light pipe is heated in order to rid condensation and maximize path length for enhanced sensitivity • This also minimizes the dead volume to lessen band broadening • Detector - highly sensitivity, liquid nitrogen cooled • Scanning is started and a brief delay is needed for compound to travel form the More on General GCIR • Very sensitive • very expensive • sample recovery Practical Uses • Pharmaceutical • Industrial • DNA Analysis of blood samples, other fluids • many others INTERFACE to Multiwavelength UV / VIS Detectors • Monitor several specific wavelengths set by colored dyes attached (DNA) • Flow through a multiwavelength detector and optical multichannel analyzer Conclusion • Gas chromatography can be effectively coupled with uv/vis detectors for monitoring dye labels, and infra-red spectroscopy and mass spectroscopy to more effectively analyze mixtures. • This is also true for liquid chromatography, although the interfaces present different problems.