Heat Transfer
advertisement

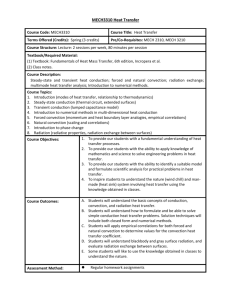
Heat Transfer Getting from here to there …Let me count the ways • Recall from the last chapter that HEAT transfers from on object to another until their TEMPERATURES are equal. • There are three ways for heat to transfer from one region to another. – Conduction – Convection – Radiation Conduction • Let’s say you have a metal spoon that you leave in a pot of boiling water for several minutes. When you grab the spoon, will you burn your hand? Why? • Now you try taping a second spoon to the handle of the first and you leave it for a few more minutes. What will happen now when you go grab the second one? • Then you give up on metal spoons and change to a wooden spoon. Would you still burn your hand if you leave the wooden spoon in the water for several minutes? Why or why not? Understanding conduction • What is was difference between the cases in the previous slide? • In the first spoon, heat was conducted within the material • With the two spoons, heat easily was conducted from the first to the second because they were in contact with one another. What conduction is • Conduction is heat transferred from particle to particle within an object or between two objects in contact. • If heat transfers easily within an object, that object is a good CONDUCTOR • If heat does not transfer easily within an object, that object is a good INSULATOR Why conduction occurs • Metals are good conductors. • Remember atoms? Remember protons and electrons? Which ones are in the nucleus and which are outside? • Conduction occurs because metals have electrons that they can lose easily. • When one end of a metal rod is heated, the atoms in that end gain energy. The electrons in that region gain enough energy to drift away from their atoms and bounce into other atoms and electrons. • This drift is the conduction of heat. Impromptu conductor/insulator quiz • • • • • • • • Steel Snow Wood Water copper Fiberglass Gold Now pick four of these and give examples of how they are used as a thermal insulator or conductor. A check for understanding • I need some volunteers… Convection • Remember that conduction was the transfer of heat (energy) from molecule to molecule within a substance. • CONVECTION is heat transfer where the molecules themselves move around. • Examples – Air currents – Boiling water Why does convection happen? • What do we know happens whenever something is heated? • It expands • If you have two objects of the same mass but different volumes, what can we say about their densities? • What happens when you have fluids of different densities? • So if you have warm air, what does it do? Why does convection happen? • So if warm air or water rises, whole volumes of air or water will physically move around. • Think about this and make sure you understand the difference between this and conduction. Candle/Test tube demo? • If the equipment is ready… Examples of convection to discuss • We are now going to break up into groups of 6. Each group will have 5 minutes to come up with and explanation for one of the following phenomena: – Land breeze/sea breeze – Why does boiling water ‘roil’? – If you light a match on earth, it burns. If you light a match on a space station, it snuffs out quickly. Why? – Why can you stay warm in an igloo even though it’s made of cold snow? Check for understanding • Once again, we need a few volunteers… • Someone has to explain why you can put your hand 2 inches away from the side of a candle flame for as long as you want, but can’t put your hand 2 inches above a candle flame for the same length of time. Radiation • IMPORTANT NOTE: Heat radiation is NOT the same thing as radioactivity. • Radiation in this case is conduction of heat by electromagnetic waves. • Give some examples of electromagnetic waves. • What is the difference among these? Wavelength http://www.qrg.northwestern.edu/projects/vss/docs/media/Communications/wavelength.gif Electromagnetic Radiation http://www.antonine-education.co.uk/physics_gcse/Unit_1/Topic_5/em_spectrum.jpg Okay, so here’s the deal… • Pretty much all objects continually give off heat by radiation. • This radiation is at mixture of different wavelengths – The cooler the object, the longer the wavelengths – The warmer the object, the shorter the wavelengths • Hotter everyday objects emit radiation in the INFRARED (pronounced “infra-red”) range • Let’s look at some videos… • Everyone write down one thing they noticed in each video Discussion of videos • What did you notice about the videos? Somebody get a rope… • K, I need some more volunteers for a demo… One last bit about radiation… • An object does two things to different amounts when it encounters radiated heat: Absorb and reflect • Absorption is when an object takes in the radiated energy • Reflection is the opposite. The radiated heat is not taken in. • Think about things that absorb and reflect light. What do you notice about these things? Final check for understanding • Once again with the volunteers
![Applied Heat Transfer [Opens in New Window]](http://s3.studylib.net/store/data/008526779_1-b12564ed87263f3384d65f395321d919-300x300.png)