6.6 THE COMPOSITION OF UNKNOWN COMPOUNDS
advertisement

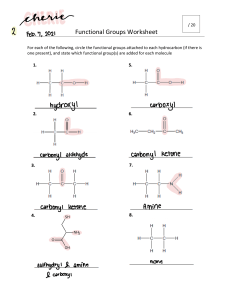
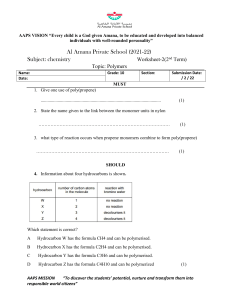
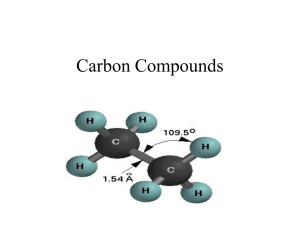
6.6 THE COMPOSITION OF UNKNOWN COMPOUNDS PERCENTAGE COMPOSITION - defined as the percentage, by mass, of each element in a compound In a 2.0g sample of a hydrocarbon, the mass of the carbon was determined to be 0.801g. What percentage of the hydrocarbon is carbon? PERCENTAGE COMPOSITION If you calculated the percent composition of a 4.0g sample, what would be your answer? This is known as THE LAW OF DEFINITE PROPORTIONS! To calculate percent composition, use the following formula: % element = melement m samplex 100% EXAMPLE 1: A 500.00mg tablet of Aspirin, C9H8O4 contains 300mg carbon and 8.08mg hydrogen. The remaining mass is oxygen. Determine its percent composition. LET'S TAKE THIS A STEP FURTHER... Let's try to calculate percentage composition from a chemical formula. EXAMPLE 2: Determine the percentage composition of calcium hydroxide, Ca(OH)2 Learning check 1. What is the percentage composition of (NH4)3PO4 N: 28% H: 8% O: 43% P: 21% HOMEWORK: Mole Airlines -due Wednesday Finish the Problem Set