Lesson 5.3 Ideal Gas Law Calculations Supplemental PV = nRT
Lesson 5.3 Ideal Gas Law Calculations Supplemental
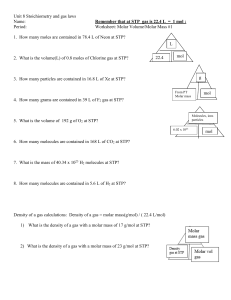
1.
2.
3.
4.
5.
6.
7.
8.
9.
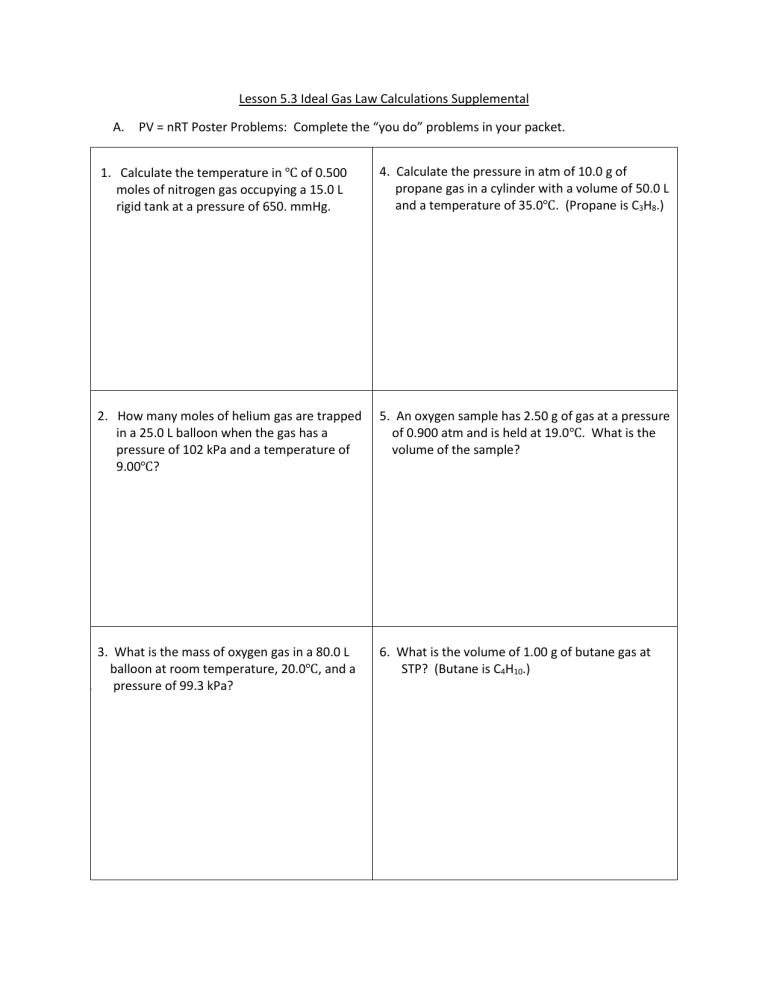
A.
PV = nRT Poster Problems: Complete the “you do” problems in your packet.
1. Calculate the temperature in ℃ of 0.500
moles of nitrogen gas occupying a 15.0 L
rigid tank at a pressure of 650. mmHg.
2. How many moles of helium gas are trapped
in a 25.0 L balloon when the gas has a
pressure of 102 kPa and a temperature of
9.00
℃
?
3. What is the mass of oxygen gas in a 80.0 L
balloon at room temperature, 20.0
℃ , and a
10.
pressure of 99.3 kPa?
4. Calculate the pressure in atm of 10.0 g of
propane gas in a cylinder with a volume of 50.0 L
and a temperature of 35.0
℃
. (Propane is C
3
H
8
.)
5. An oxygen sample has 2.50 g of gas at a pressure
of 0.900 atm and is held at 19.0
℃ . What is the
volume of the sample?
6. What is the volume of 1.00 g of butane gas at
STP? (Butane is C
4
H
10
.)
B.
PV = nRT Problems involving Molar Mass and Density
Because the ideal gas involves variables of moles and volume, you can rearrange the equation to solve for molar mass (remember: that’s mass divided by # of moles) or for density (remember: that’s mass divided by volume). Here’s the proof:
Density = Molar mass =
Practice Problems
7. Calculate the density a gas will have at STP if its molar mass is 28.7 g/mol. (1.28 g/L)
8. Uranium hexafluoride, UF
4
, is a solid at room temperature, but it boils at 56 o
C. Determine the density of uranium hexafluoride at 60 o
C and 745 mmHg. (11.3 g/L)
9. Calculate the molar mass of a gas if 35.44 g of the gas stored in a 7.50 L tank exerts a pressure of
60.0 atm at a constant temperature of 35.5 °C. (1.99 g/mol)
10. What is the molar mass of a gas if 273 mL of the gas weighs 0.80 grams at 27 o
C and 380 torr?
(144. g/mol)