CH 11 Notes - EHS

Chapter 11
Liquids, Solids, and Intermolecular
Forces
Climbing Geckos
• Geckos can adhere to almost any surface
• Recent studies indicate that this amazing ability is related to intermolecular attractive forces
• Geckos have millions of tiny hairs on their feet that branch out and flatten out on the end
– setae = hairs, spatulae = flat ends
• This structure allows the gecko to have unusually close contact to the surface – allowing the intermolecular attractive forces to act strongly
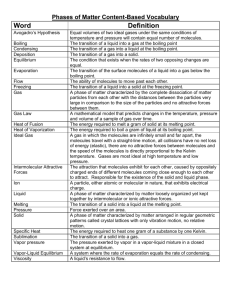
Properties of the three Phases of Matter
State Shape Volume
C om pr
W es sib ill
Density it le
?
A
Flo ttr ac w
?
In te tio rm ns
S tre ole ng th cu la
o f r
Solid fixed fixed
Liquid indefinite fixed
Gas indefinite indefinite high high low
No No
No Yes
Yes Yes very strong intermediate weak
• Fixed = keeps shape when placed in a container
• Indefinite = takes the shape of the container
Three Phases of Water
Notice that the densities of ice and liquid water are much larger than the density of steam
Notice that the densities and molar volumes of ice and liquid water are much closer to each other than to steam
Notice that the densities of ice is larger than the density of liquid water. This is not the norm, but is vital to the development of life as we know it.
Degrees of Freedom
• Particles may have one or several types of freedom of motion
– and various degrees of each type
• Translational freedom is the ability to move from one position in space to another
• Rotational freedom is the ability to reorient the particles direction in space
• Vibrational freedom is the ability to oscillate about a particular point in space
Solids
• Particles packed close and are fixed
– though they may vibrate
• Incompressible
• Retention of shape and volume
• Do not flow
• Crystalline Solids particles arranged in an orderly geometric pattern
– salt and diamonds
• Amorphous Solids – particles do not show a regular geometric pattern over a long range plastic and glass
Liquids
• Particles are closely packed, but they have some ability to move around
• Incompressible
• Take the shape of their container and to flow
• Do not have enough freedom to escape or expand to fill the container
Gases
• Particles have complete freedom of motion and are not held together
• Particles in constant motion, bumping into each other and the container
• There is a large amount of space between the particles
– compared to the size of the particles
– molar volume of the gas state of a material is much larger than the molar volume of the solid or liquid states
• Compressible
• Expand to fill and take the shape of their container, and will flow
Compressibility
Kinetic – Molecular Theory
• What state a material is in depends largely on two major factors
1. the amount of kinetic energy the particles possess
2. the strength of attraction between the particles
• These two factors are in competition with each other
States and Degrees of Freedom
• The molecules in a gas have complete freedom of motion
– their kinetic energy overcomes the attractive forces between the molecules
• The molecules in a solid are locked in place, they cannot move around
– though they do vibrate, they don ’ t have enough kinetic energy to overcome the attractive forces
• The molecules in a liquid have limited freedom – they can move around a little within the structure of the liquid
– they have enough kinetic energy to overcome some of the attractive forces, but not enough to escape each other
Kinetic Energy
• Increasing kinetic energy increases the motion energy of the particles
• The more motion energy the molecules have, the more freedom they can have
• The average kinetic energy is directly proportional to the temperature
– KE avg
= 1.5 kT
Attractive Forces
• The particles are attracted to each other by electrostatic forces
• The strength of the attractive forces varies, some are small and some are large
• The strength of the attractive forces depends on the kind(s) of particles
• The stronger the attractive forces between the particles, the more they resist moving
– though no material completely lacks particle motion
Kinetic–Molecular Theory of Gases
• When the kinetic energy is so large it overcomes the attractions between particles, the material will be a gas
• In an ideal gas, the particles have complete freedom of motion – especially translational
• This allows gas particles to expand to fill their container
– gases flow
• It also leads to there being large spaces between the particles
– therefore low density and compressibility
Gas Structure
Gas molecules are rapidly moving in random straight lines, and are free from sticking to each other.
Kinetic–Molecular Theory of Solids
• Solids exhibit strong attractive forces limiting the kinetic energy of the particles
• Solid packing results in no translational or rotational motion
– the only freedom they have is vibrational motion
Kinetic–Molecular Theory of Liquids
• Liquids exhibit attractive forces strong enough to partially overcome the kinetic energy
• Particles are packed together with only very limited translational or rotational freedom
Explaining the Properties of Liquids
• Because the particles are in contact…
– Higher densities than gases
– Incompressible
• Because the particles have limited translational freedom…
– Indefinite shape allowing for flow
– Take the shape of the container
• Because the limit on their freedom keeps the particles from escaping each other
– Liquids have a definite volume
Phase Changes
• The attractive forces between the molecules are fixed changing the material’s state requires changing the amount of kinetic energy that is limiting the particles freedom
– Solids melt when heated because the particles gain enough kinetic energy to partially overcome the attractive forces
– Liquids boil when heated because the particles gain enough kinetic energy to completely overcome the attractive forces
– the stronger the attractive forces, the higher you will need to raise the temperature
• Gases can be condensed by decreasing the temperature and/or increasing the pressure
– pressure can be increased by decreasing the gas volume
– reducing the volume reduces the amount of translational freedom the particles have
Phase Changes
Intermolecular Attractions
• The strength of the attractions between the particles of a substance determines its state
• At room temperature, moderate to strong attractive forces result in materials being solids or liquids
• The stronger the attractive forces are, the higher will be the boiling point of the liquid and the melting point of the solid
– other factors also influence the melting point
Why Are Molecules Attracted to Each Other?
• Intermolecular attractions are due to attractive forces between opposite charges
– + ion to − ion
– + end of polar molecule to − end of polar molecule
• H-bonding especially strong
– even nonpolar molecules will have temporary charges
• Larger charge = stronger attraction
• Longer distance = weaker attraction
• However, these attractive forces are small relative to the bonding forces between atoms
– generally smaller charges
– generally over much larger distances
Trends in the Strength of
Intermolecular Attraction
• The stronger the attractions between the atoms or molecules, the more energy it will take to separate them
• Boiling a liquid requires we add enough energy to overcome all the attractions between the particles
– However, not breaking the covalent bonds
• The higher the normal boiling point of the liquid, the stronger the intermolecular attractive forces
Kinds of Attractive Forces
• Dispersion Forces Unequal electron distribution leads to attractions causing temporary polarity in the molecules
• Dipole–Dipole Attractions Permanent polarity in the molecules due to their structure leads to attractive forces
• Hydrogen Bonds - An especially strong dipole– dipole attraction resulting when H is attached to an extremely electronegative atom.
Dispersion Forces
• Fluctuations in the electron distribution in atoms and molecules result in a temporary dipole
– region with excess electron density has partial (─) charge
– region with depleted electron density has partial (+) charge
• The attractive forces caused by these temporary dipoles are called dispersion forces
– aka London Forces
• All molecules and atoms will have them
• As a temporary dipole is established in one molecule, it induces a dipole in all the surrounding molecules
Dispersion Force
Size of the Induced Dipole
• The magnitude of the induced dipole depends on several factors + +
+ + + +
• Polarizability of the electrons
- -
-
- -
– volume of the electron cloud
+
– larger molar mass = more electrons = larger electron cloud = increased polarizability = stronger attractions
• Shape of the molecule
– more surface-to-surface contact = larger induced dipole = stronger attraction +
+
+ spherical ones
+
+ +
+ + +
+
+
+
+
−
−
−
− − −
-
-
−
−
−
Effect of Molecular Size on Size of Dispersion Force
Therefore the strength of the dispersion forces increases.
The stronger the attractive forces between the molecules, the higher the boiling point will be.
Properties of Straight Chain Alkanes
NonPolar Molecules
Boiling Points of n-Alkanes
Effect of Molecular Shape on Size of Dispersion Force
the larger surface-tosurface contact between molecules in n -pentane results in stronger dispersion force attractions
Alkane Boiling Points
• Branched chains have lower BPs than straight chains
• The straight chain isomers have more surface-tosurface contact
Practice – Choose the Substance in Each Pair with the Higher Boiling Point a) CH
4
C
4
H
10 b) C
6
H
12
C
6
H
12
Practice – Choose the Substance in Each Pair with the Higher Boiling Point a) CH
4
CH
3
CH
2
CH
2
CH
3
Both molecules are nonpolar larger molar mass b) CH
3
CH
2
CH=CHCH
2
CH
3 cyclohexane
Both molecules are nonpolar, but the flatter ring molecule has larger surface-tosurface contact
Dipole–Dipole Attractions
• Polar molecules have a permanent dipole
– because of bond polarity and shape
– dipole moment
– as well as the always present induced dipole
• The permanent dipole adds to the attractive forces between the molecules
– raising the boiling and melting points relative to nonpolar molecules of similar size and shape
Effect of Dipole–Dipole Attraction on
Boiling and Melting Points
replace with the figure 11.8
Example 11.1b: Determine if dipole–dipole attractions occur between CH
2
Cl
2 molecules
Given:
Find:
CH
2
Cl
2
, EN C = 2.5, H = 2.1, Cl = 3.0
If there are dipole –dipole attractions
Conceptual Plan:
Relationships:
Formula
Lewis
Structure
Bond
Polarity
EN Difference Shape
Molecule
Polarity molecules that have dipole
–dipole attractions must be polar
Solution: Cl —C polar bonds and tetrahedral shape
= polar molecule polar tetrahedral shape
C —H
2.5−2.1 = 0.4
nonpolar polar molecule; therefore dipole – dipole attractions
Practice – Choose the substance in each pair with the higher boiling point a)CH
2
FCH
2
F CH
3
CHF
2 b) or
Practice – Choose the substance in each pair with the higher boiling point a)CH
2
FCH
2
F CH
3
CHF
2 more polar b) polar or nonpolar
Hydrogen Bonding
• When a very electronegative atom is bonded to hydrogen, it strongly pulls the bonding electrons toward it
– O ─ H, N ─ H, or F ─ H
• Because hydrogen has no other electrons, when its electron is pulled away, the nucleus becomes deshielded
– exposing the H proton
• The exposed proton acts as a very strong center of positive charge, attracting all the electron clouds from neighboring molecules
H-Bonding
HF
H-Bonding in Water
H-Bonds
• Hydrogen bonds are very strong intermolecular attractive forces
– stronger than dipole–dipole or dispersion forces
• Substances that can hydrogen bond will have higher boiling points and melting points than similar substances that cannot
• But hydrogen bonds are not nearly as strong as chemical bonds
– 2 to 5% the strength of covalent bonds
Effect of H-Bonding on Boiling Point
Polar molecules, such as the hydrides of Groups 5 –7, have both dispersion forces and dipole –dipole attractions. Therefore they have higher boiling points than the corresponding Group 4 molecules.
Example 11.2: Which of these compounds is a liquid at room temperature (the others are gases). Why?
MM = 30.03
Polar
No H-Bonds
MM = 34.03
Polar
No H-Bonds
MM = 34.02
Polar
H-Bonds substance with the strongest will be the liquid.
dispersion forces: MM 30.03, trigonal planar
Because only hydrogen peroxide has the additional very dipole strong H -
–dipole: polar O H bonds uncancelled bond additional attractions, its intermolecular
H-bonding: no O H, N H, or F –H therefore no H attractions will be the strongest. We therefore expect
bond
Practice – Choose the substance in each pair that is a liquid at room temperature (the other is a gas) a) CH
3
OH CH
3
CHF
2 can H-bond b) CH
3
-O-CH
2
CH
3
CH
3
CH
2
CH
2
NH
2 can H-bond
Attractive Forces and Solubility
• Solubility depends, in part, on the attractive forces of the solute and solvent molecules
– like dissolves like
– miscible liquids will always dissolve in each other
• Polar substances dissolve in polar solvents
– hydrophilic groups = OH, CHO, C=O, COOH, NH
2
, Cl
• Nonpolar molecules dissolve in nonpolar solvents
– hydrophobic groups = C-H, C-C
• Many molecules have both hydrophilic and hydrophobic parts – solubility in water becomes a competition between the attraction of the polar groups for the water and the attraction of the nonpolar groups for their own kind
Pentane, C
5 molecule.
H
12
Immiscible Liquids
is a nonpolar
Water is a polar molecule.
The attractive forces between the water molecules is much stronger than their attractions for the pentane molecules. The result is the liquids are immiscible.
Polar Solvents
Dichloromethane
(methylene chloride)
Water
Ethanol
(ethyl alcohol)
Nonpolar Solvents
Ion–Dipole Attraction
• In a mixture, ions from an ionic compound are attracted to the dipole of polar molecules
• The strength of the ion–dipole attraction is one of the main factors that determines the solubility of ionic compounds in water
Practice – Choose the substance in each pair that is more soluble in water a) CH
3
OH can H-bond with H
2
O
CH
3
CHF
2 b) CH
3
CH
2
CH
2
CH
2
CH
3
CH
3
Cl more polar
Summary
• Dispersion forces
– The weakest of the intermolecular attractions
– Present in all molecules and atoms
– Magnitude of the dispersion forces increases with molar mass
– Polar molecules also have dipole–dipole attractive forces
• Hydrogen bonds
– the strongest of the intermolecular attractive forces a pure substance can have
– present when a molecule has H directly bonded to either O , N, or F atoms
• only example of H bonded to F is HF
• Ion–dipole attractions
– present in mixtures of ionic compounds with polar molecules.
– the strongest intermolecular attraction
– especially important in aqueous solutions of ionic compounds
Liquids
Properties & Structure
Surface Tension
• Surface tension is a property of liquids that results from the tendency of liquids to minimize their surface area
• To minimize their surface area, liquids form drops that are spherical
– as long as there is no gravity
Surface Tension
• The layer of molecules on the surface behave differently than molecules in the interior
– the cohesive forces on the surface molecules have a net pull into the liquid interior
– The surface layer acts like an elastic skin allowing you to “ float ” a paper clip even though steel is denser than water
• Surface molecules have a higher potential energy and are less stable than those in the interior because they have fewer neighbors to attract them
• The surface tension of a liquid is the energy required to increase the surface area a given amount
– surface tension of H
2
O = 72.8 mJ/m
• at room temperature
2
– surface tension of C
6
H
6
= 28 mJ/m 2
Factors Affecting Surface Tension
• Stronger intermolecular attractive forces higher surface tension
• Raising the temperature of a liquid reduces its surface tension
– increases the average kinetic energy of the molecules
– the increased molecular motion makes it easier to stretch the surface
Viscosity
• Viscosity is the resistance of a liquid to flow
– 1 poise = 1 P = 1 g/cm∙s
– often given in centipoise, cP
• H
2
O = 1 cP at room temperature
• Larger intermolecular attractions = larger viscosity
Factors Affecting
Viscosity
• The stronger the intermolecular attractive forces, the higher the liquid ’ s viscosity
• The more spherical the molecular shape, the lower the viscosity will be
– molecules roll more easily
– less surface-to-surface contact lowers attractions
• Raising the temperature of a liquid reduces its viscosity
– raising the temperature of the liquid increases the average kinetic energy of the molecules
– the increased molecular motion makes it easier to overcome the intermolecular attractions and flow
Insert Table 11.6
Capillary Action
• Capillary action is the ability of a liquid to flow up a thin tube against the influence of gravity
– the narrower the tube, the higher the liquid rises
• Capillary action is the result of two forces working in conjunction, the cohesive and adhesive forces
– cohesive forces hold the liquid molecules together
– adhesive forces attract the outer liquid molecules to the tube ’ s surface
Capillary Action
• The adhesive forces pull the surface liquid up the side of the tube, and the cohesive forces pull the interior liquid with it
• The liquid rises up the tube until the force of gravity counteracts the capillary action forces
• The narrower the tube diameter, the higher the liquid will rise up the tube
Meniscus
• The curving of the liquid surface in a thin tube is due to the competition between adhesive and cohesive forces
• The meniscus of water (dyed red here) is concave in a glass tube because its adhesion to the glass is stronger than its cohesion for itself
• The meniscus of mercury is convex in a glass tube because its cohesion for itself is stronger than its adhesion for the glass
– metallic bonds are stronger than intermolecular attractions
The Molecular Dance
• Molecules in the liquid are constantly in motion
– vibrational, and limited rotational and translational
• The average kinetic energy is proportional to the temperature
• However, some molecules have more kinetic energy than the average, and others have less
Vaporization
• If these high energy molecules are at the surface, they may have enough energy to overcome the attractive forces
– therefore – the larger the surface area, the faster the rate of evaporation
• This will allow them to escape the liquid and become a vapor
Distribution of Thermal Energy
• Only a small fraction of the molecules in a liquid have enough energy to escape
• But, as the temperature increases, the fraction of the molecules with “ escape energy ” increases
• The higher the temperature, the faster the rate of evaporation
Condensation
• Some molecules of the vapor will lose energy through molecular collisions
• The result will be that some of the molecules will get captured back into the liquid when they collide with it
• Also some may stick and gather together to form droplets of liquid
– particularly on surrounding surfaces
• We call this process condensation
Evaporation vs. Condensation
• Vaporization and condensation are opposite processes
• In an open container, the vapor molecules generally spread out faster than they can condense
• The net result is that the rate of vaporization is greater than the rate of condensation, and there is a net loss of liquid
• However, in a closed container, the vapor is not allowed to spread out indefinitely
• The net result in a closed container is that at some time the rates of vaporization and condensation will be equal
Effect of Intermolecular Attraction on
Evaporation and Condensation
• The weaker the attractive forces between molecules, the less energy they will need to vaporize
• Also, weaker attractive forces means that more energy will need to be removed from the vapor molecules before they can condense
• The net result will be more molecules in the vapor phase, and a liquid that evaporates faster – the weaker the attractive forces, the faster the rate of evaporation
• Liquids that evaporate easily are said to be volatile
– e.g., gasoline, fingernail polish remover
– liquids that do not evaporate easily are called nonvolatile
• e.g., motor oil
Energetics of Vaporization
• When the high energy molecules are lost from the liquid, it lowers the average kinetic energy
• If energy is not drawn back into the liquid, its temperature will decrease – therefore, vaporization is an endothermic process
– and condensation is an exothermic process
• Vaporization requires input of energy to overcome the attractions between molecules
Heat of Vaporization
• The amount of heat energy required to vaporize one mole of the liquid is called the heat of vaporization,
D
H vap
– sometimes called the enthalpy of vaporization
• Always endothermic, therefore
D
H vap is +
• Somewhat temperature dependent
D
H condensation
= −
D
H vaporization
Example 11.3: Calculate the mass of water that can be vaporized with 155 kJ of heat at 100 °C
Given:
Find:
155 kJ g H
2
O
Conceptual Plan: kJ mol H
2
O
Relationships:
1 mol H
2
O = 40.7 kJ, 1 mol = 18.02 g g H
2
O
Solution:
Check: because the given amount of heat is almost 4x the
D
H vap
, the amount of water makes sense
Practice – Calculate the amount of heat needed to vaporize 90.0 g of C
3
H
7
OH at its boiling point
(
D
H vap
= 39.9 kJ/mol)
Practice – Calculate the amount of heat needed to vaporize 90.0 g of C
3
H
7
OH at its boiling point
Given:
Find:
90.0 g kJ
Conceptual Plan:
Relationships: g mol kJ
1 mol C
3
H
7
OH = 39.9 kJ, 1 mol = 60.09 g
Solution:
Check: because the given amount of C
3
H
7
OH is more than 1 mole the amount of heat makes sense
Dynamic Equilibrium
• In a closed container, once the rates of vaporization and condensation are equal, the total amount of vapor and liquid will not change
• Evaporation and condensation are still occurring, but because they are opposite processes, there is no net gain or loss of either vapor or liquid
• When two opposite processes reach the same rate so that there is no gain or loss of material, we call it a dynamic equilibrium
– this does not mean there are equal amounts of vapor and liquid – it means that they are changing by equal amounts
Dynamic Equilibrium
Vapor Pressure
• The pressure exerted by the vapor when it is in dynamic equilibrium with its liquid is called the vapor pressure
– remember using Dalton ’ s Law of Partial Pressures to account for the pressure of the water vapor when collecting gases by water displacement?
• The weaker the attractive forces between the molecules, the more molecules will be in the vapor
• Therefore, the weaker the attractive forces, the higher the vapor pressure
– the higher the vapor pressure, the more volatile the liquid
Vapor–Liquid Dynamic Equilibrium
• If the volume of the chamber is increased, it will decrease the pressure of the vapor inside the chamber
– at that point, there are fewer vapor molecules in a given volume, causing the rate of condensation to slow
• Therefore, for a period of time, the rate of vaporization will be faster than the rate of condensation, and the amount of vapor will increase
• Eventually enough vapor accumulates so that the rate of the condensation increases to the point where it is once again as fast as evaporation
– equilibrium is reestablished
• At this point, the vapor pressure will be the same as it was before
Changing the Container ’ s Volume
Disturbs the Equilibrium
Initially, the rate of vaporization and condensation are equal and the system is in dynamic equilibrium
When the volume is increased, the rate of vaporization becomes faster than the rate of condensation
When the volume is decreased, the rate of vaporization becomes slower than the rate of condensation
Dynamic Equilibrium
• A system in dynamic equilibrium can respond to changes in the conditions
• When conditions change, the system shifts its position to relieve or reduce the effects of the change
Vapor Pressure vs. Temperature
• Increasing the temperature increases the number of molecules able to escape the liquid
• The net result is that as the temperature increases, the vapor pressure increases
• Small changes in temperature can make big changes in vapor pressure
– the rate of growth depends on strength of the intermolecular forces
Vapor Pressure Curves
normal BP
100 ° C
760 mmHg
BP Ethanol at 500 mmHg
68.1
° C
Practice – Which of the following is the most volatile?
Practice – Which of the following has the strongest Intermolecular attractions?
Boiling Point
• When the temperature of a liquid reaches a point where its vapor pressure is the same as the external pressure, vapor bubbles can form anywhere in the liquid
– not just on the surface
• This phenomenon is what is called boiling and the temperature at which the vapor pressure = external pressure is the boiling point
Boiling Point
• The normal boiling point is the temperature at which the vapor pressure of the liquid = 1 atm
• The lower the external pressure, the lower the boiling point of the liquid
Practice – Which of the following has the highest normal boiling point?
Heating Curve of a Liquid
• As you heat a liquid, its temperature increases linearly until it reaches the boiling point
– q = mass x
C s x D
T
• Once the temperature reaches the boiling point, all the added heat goes into boiling the liquid – the temperature stays constant
• Once all the liquid has been turned into gas, the temperature can again start to rise
Clausius–Clapeyron Equation
•
• A graph of ln(P vap
) vs. 1/T is a straight line
The graph of vapor pressure vs. temperature is an vap
Example 11.4: Determine the
D
H vap of dichloromethane given the vapor pressure vs. temperature data
• Enter the data into a spreadsheet and calculate the inverse of the absolute temperature and natural log of the vapor pressure
Example 11.4: Determine the
D
H vap of dichloromethane given the vapor pressure vs. temperature data
• Graph the inverse of the absolute temperature vs. the natural log of the vapor pressure
Example 11.4: Determine the
D
H vap of dichloromethane given the vapor pressure vs. temperature data
• Add a trendline, making sure the display equation on chart option is checked off
Example 11.4: Determine the
D
H vap of dichloromethane given the vapor pressure vs. temperature data
• Determine the slope of the line
– −3776.7 ≈ −3800 K
Example 11.4: Determine the
D
H vap of dichloromethane given the vapor pressure vs. temperature data
• Use the slope of the line to determine the heat of vaporization
– slope ≈ −3800 K, R = 8.314 J/mol∙K
Clausius–Clapeyron Equation
2-Point Form
• The equation below can be used with just two measurements of vapor pressure and temperature
– however, it generally gives less precise results
• fewer data points will not give as precise an average because there is less averaging out of the errors o as with any other sets of measurements
• It can also be used to predict the vapor pressure if you know the heat of vaporization and the normal boiling point
– remember: the vapor pressure at the normal boiling point is 760 torr
Example 11.5: Calculate the vapor pressure of
Given: methanol at 12.0 °C
= BP = 64.6 °C, P = 760 torr,
D
H
= 12.0 °C
Find:
Conceptual
Plan:
P
1
, T
1
,
D
H vap
P
2
Relationships:
T(K) = T( ° C) + 273.15
Solution:
Check: the units are correct, the size makes sense because the vapor pressure is lower at lower temperatures
Practice – Determine the vapor pressure of water at 25
C (normal BP = 100.0
C,
D
H vap
= 40.7 kJ/mol)
Practice – Determine the vapor pressure of water at
25
C
Given: = BP = 100.0 °C, P
1
= 25.0 °C
= 760 torr,
D
H vap
Find:
1 1
2 2
P
2 2
, torr
Conceptual Plan:
P
1
, T
1
,
D
H vap
P
2
Relationships:
Solution:
T(K) = T( ° C) + 273.15
Check: the units are correct, the size makes sense because the vapor pressure is lower at lower temperatures
Supercritical Fluid
• As a liquid is heated in a sealed container, more vapor collects, causing the pressure inside the container to rise
– and the density of the vapor to increase
– and the density of the liquid to decrease
• At some temperature, the meniscus between the liquid and vapor disappears and the states commingle to form a supercritical fluid
• Supercritical fluids have properties of both gas and liquid states
The Critical Point
• The temperature required to produce a supercritical fluid is called the critical temperature
• The pressure at the critical temperature is called the critical pressure
• At the critical temperature or higher temperatures, the gas cannot be condensed to a liquid, no matter how high the pressure gets
Sublimation and Deposition
• Molecules in the solid have thermal energy that allows them to vibrate
• Surface molecules with sufficient energy may break free from the surface and become a gas – this process is called sublimation
• The capturing of vapor molecules into a solid is called deposition
• The solid and vapor phases exist in dynamic equilibrium in a closed container
– at temperatures below the melting point
– therefore, molecular solids have a vapor pressure sublimation
Sublimation & Deposition
Melting = Fusion
• As a solid is heated, its temperature rises and the molecules vibrate more vigorously
• Once the temperature reaches the melting point, the molecules have sufficient energy to overcome some of the attractions that hold them in position and the solid melts (or fuses)
• The opposite of melting is freezing
Heating Curve of a Solid
• As you heat a solid, its temperature increases linearly until it reaches the melting point
– q = mass x
C s x D
T
• Once the temperature reaches the melting point, all the added heat goes into melting the solid – the temperature stays constant
• Once all the solid has been turned into liquid, the temperature can again start to rise
– ice/water will always have a temperature of 0 °C
• at 1 atm
Energetics of Melting
• When the high energy molecules are lost from the solid, it lowers the average kinetic energy
• If energy is not drawn back into the solid its temperature will decrease – therefore, melting is an endothermic process
– and freezing is an exothermic process
• Melting requires input of energy to overcome the attractions between molecules
Heat of Fusion
• The amount of heat energy required to melt one mole of the solid is called the Heat of Fusion,
D
H fus
– sometimes called the enthalpy of fusion
• Always endothermic, therefore
D
H fus
• Somewhat temperature dependent
D
H crystallization
= −
D
H fusion
Generally much less than
D
H
D
H sublimation
=
D
H fusion vap
+
D
H vaporization is +
Heats of Fusion and Vaporization
Heating Curve of Water
Segment 1
•
• Heating 1.00 mole of ice at −25.0 °C up to the melting point, 0.0 °C
q = mass x
C s x D
T
– mass of 1.00 mole of ice = 18.0 g
– C s
= 2.09 J/mol∙°C
Segment 2
• Melting 1.00 mole of ice at the melting point, 0.0 °C
• q = n∙
D
H fus
– n = 1.00 mole of ice
– D
H fus
= 6.02 kJ/mol
Segment 3
•
• Heating 1.00 mole of water at 0.0 °C up to the boiling point, 100.0 °C
q = mass x
C s x D
T
– mass of 1.00 mole of water = 18.0 g
– C s
= 2.09 J/mol∙°C
Segment 4
• Boiling 1.00 mole of water at the boiling point, 100.0 °C
• q = n∙
D
H vap
– n = 1.00 mole of ice
– D
H fus
= 40.7 kJ/mol
Segment 5
• Heating 1.00 mole of steam at 100.0 °C up to 125.0 °C
• q = mass x
C s x D
T
– mass of 1.00 mole of water = 18.0 g
– C s
= 2.01 J/mol∙°C
Practice – How much heat, in kJ, is needed to raise the temperature of a 12.0 g benzene sample from −10.0 °C to 25.0 °C?
Practice – How much heat is needed to raise the temperature of a
12.0 g benzene sample from −10.0 °C to 25.0 °C?
Given:
Find:
12.0 g benzene, seg 1 = 0.2325 kJ,
2
= 5.5 °C, T
= 5.5
= 25.0
°C),
°C)
Conceptual Plan: g
Relationships:
D
H
C s fus
9.8 kJ/mol, 1 mol = 78.11 g, 1 kJ = 1000 J, q = m∙C s
∙ D
T
,sol = 1.25 J/g °C, C s
,liq = 1.70 J/g °C
Solution:
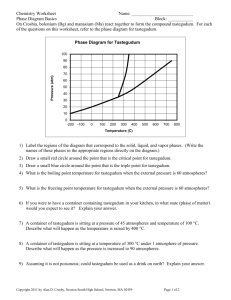
Phase Diagrams
• Phase diagrams describe the different states and state changes that occur at various temperature/pressure conditions
• Regions represent states
• Lines represent state changes
– liquid/gas line is vapor pressure curve
– both states exist simultaneously
– critical point is the furthest point on the vapor pressure curve
• Triple point is the temperature/pressure condition where all three states exist simultaneously
• For most substances, freezing point increases as pressure increases
1 atm
Solid melting
Phase Diagrams
Fusion Curve freezing
Liquid normal melting pt.
critical point normal boiling pt.
Sublimation
Curve triple point vaporization condensation Vapor Pressure
Curve sublimation deposition
Gas
Temperature
1 atm
Ice
Phase Diagram of Water
critical point
374.1
°C
217.7 atm
Water normal melting pt.
0 °C normal boiling pt.
100 °C triple point
0.01 °C
0.006 atm
Steam
Temperature
Morphic Forms of Ice
Solid
Phase Diagram of CO
2 critical point
31.0 °C
72.9 atm
Liquid
-56.7 °C
5.1 atm
1 atm normal sublimation pt.
-78.5 °C triple point
Gas
Temperature
Practice – Consider the phase diagram of CO
2 shown. What phase(s) is/are present at each of the following conditions?
• • 20.0 ° C, 72.9 atm liquid
• • −56.7 ° C, 5.1 atm solid, liquid, gas
• • 10.0 ° C, 1.0 atm gas
• • −78.5 ° C, 1.0 atm solid, gas
• • 50.0 ° C, 80.0 atm scf
Water – An Extraordinary Substance
• Water is a liquid at room temperature
– most molecular substances with similar molar masses are gases at room temperature
• e.g. NH
3
, CH
4
– due to H-bonding between molecules
• Water is an excellent solvent – dissolving many ionic and polar molecular substances
– because of its large dipole moment
– even many small nonpolar molecules have some solubility in water
• e.g. O
2
, CO
2
• Water has a very high specific heat for a molecular substance
– moderating effect on coastal climates
• Water expands when it freezes
• at a pressure of 1 atm
– about 9%
– making ice less dense than liquid water
Solids
Properties &
Structure
Crystal Lattice
• When allowed to cool slowly, the particles in a liquid will arrange themselves to give the maximum attractive forces
– therefore minimize the energy
• The result will generally be a crystalline solid
• The arrangement of the particles in a crystalline solid is called the crystal lattice
• The smallest unit that shows the pattern of arrangement for all the particles is called the unit cell
Unit Cells
• Unit cells are 3-dimensional
– usually containing 2 or 3 layers of particles
• Unit cells are repeated over and over to give the macroscopic crystal structure of the solid
• Starting anywhere within the crystal results in the same unit cell
• Each particle in the unit cell is called a lattice point
• Lattice planes are planes connecting equivalent points in unit cells throughout the lattice
a
Cubic a = b = c all 90 ° c b c b a
Hexagonal a = c < b
2 faces 90 °
1 face 120 °
7 Unit Cells
c c b b a
Tetragonal a = c < b all 90 ° a
Orthorhombic a
b
c all 90 ° b a
Rhombohedral a = b = c no 90 ° c c a
Triclinic a
b
c no 90 ° c b a b
Monoclinic a
b
c
2 faces 90 °
Unit Cells
• The number of other particles each particle is in contact with is called its coordination number
– for ions, it is the number of oppositely charged ions an ion is in contact with
• Higher coordination number means more interaction, therefore stronger attractive forces holding the crystal together
• The packing efficiency is the percentage of volume in the unit cell occupied by particles
– the higher the coordination number, the more efficiently the particles are packing together
Cubic Unit Cells
• All 90° angles between corners of the unit cell
• The length of all the edges are equal
• If the unit cell is made of spherical particles
– ⅛ of each corner particle is within the cube
– ½ of each particle on a face is within the cube
– ¼ of each particle on an edge is within the cube
Cubic Unit Cells -
Simple Cubic
• Eight particles, one at each corner of a cube
• 1/8 th of each particle lies in the unit cell
– each particle part of eight cells
– total = one particle in each unit cell
• 8 corners x 1/8
• Edge of unit cell = twice the radius
• Coordination number of 6
2r
Simple Cubic
Cubic Unit Cells -
Body-Centered Cubic
• Nine particles, one at each corner of a cube + one in center
• 1/8 th of each corner particle lies in the unit cell
– two particles in each unit cell
• 8 corners x 1/8
• + 1 center
• Edge of unit cell = (4/
3) times the radius of the particle
• Coordination number of 8
4 r
3
Body-Centered Cubic
Cubic Unit Cells -
Face-Centered Cubic
• 14 particles, one at each corner of a cube + one in center of each face
• 1/8 th of each corner particle + 1/2 of face particle lies in the unit cell
– 4 particles in each unit cell
• 8 corners x 1/8
• + 6 faces x 1/2
• Edge of unit cell = 2 2 times the radius of the particle
• Coordination number of 12
2 r 2
Face-Centered Cubic
Example 11.6: Calculate the density of Al if it crystallizes in a fcc and has a radius of 143 pm
Given: face-centered cubic, V r = 6.618 x 10 g g
Find: density, g/cm 3
Conceptual
Plan: fcc mass
# atoms x mass of 1 atom r l l = 2 r √2 V = l 3
V
Relationships: m, V d d = m /V
1 cm = 10 2 m, 1 pm = 10
−12 m V = l 3 , l = 2 r
√2, d = m /V fcc = 4 atoms/uc, Al = 26.982 g/mol, 1 mol = 6.022 x 10 23 atoms
Solution:
Check: the accepted density of Al at 20 °C is 2.71 g/cm 3 , so the answer makes sense
Practice – Estimate the density of Rb if it crystallizes in a body-centered cubic unit cell and has an atomic radius of
247.5 pm
Practice – Estimate the density of Rb if it crystallizes in a bcc and has a radius of 247.5 pm
Given:
Find: body-centered cubic, r r = 247.5 pm cm, m = 2.839 x 10 g g density, g/cm 3 3
Conceptual
Plan: bcc mass
# atoms x mass of 1 atom r l l = 4 r/ √3 V = l 3
V
Relationships: m, V d d = m /V
1 cm = 10 2 m, 1 pm = 10
−12 m V = l 3 , l = 4 r/
√3, d = m /V bcc = 2 atoms/uc, Rb = 85.47 g/mol, 1 mol = 6.022 x 10 23 atoms
Solution:
Check: the accepted density of Rb at 20 °C is 1.53 g/cm 3 , so the answer makes sense
Closest-Packed Structures
First Layer
• With spheres, it is more efficient to offset each row in the gaps of the previous row than to line up rows and columns
Closest-Packed Structures
Second Layer
• The second layer atoms can sit directly over the atoms in the first layer– called an AA pattern
• Or the second layer can sit over the holes in the first layer – called an AB pattern
Closest-Packed Structures
Third Layer – with Offset 2
nd
Layer
• The third layer atoms can align directly over the atoms in the first layer– called an ABA pattern
• Or the third layer can sit over the uncovered holes in the first layer – called an ABC pattern
Face-Centered Cubic
Hexagonal Closest-Packed Structures
Cubic Closest-Packed Structures
Classifying Crystalline Solids
• Crystalline solids are classified by the kinds of particles found
• Some of the categories are sub-classified by the kinds of attractive forces holding the particles together
Classifying Crystalline Solids
• Molecular solids are solids whose composite particles are molecules
• Ionic solids are solids whose composite particles are ions
• Atomic solids are solids whose composite particles are atoms
– nonbonding atomic solids are held together by dispersion forces
– metallic atomic solids are held together by metallic bonds
– network covalent atomic solids are held together by covalent bonds
Molecular Solids
• The lattice sites are occupied by molecules
– CO
2
, H
2
O, C
12
H
22
O
11
• The molecules are held together by intermolecular attractive forces
– dispersion forces, dipole–dipole attractions, and Hbonds
• Because the attractive forces are weak, they tend to have low melting points
– generally < 300 °C
Ionic Solids
• Lattice sites occupied by ions
• Held together by attractions between oppositely charged ions
– nondirectional
– therefore every cation attracts all anions around it, and vice-versa
• The coordination number represents the number of close cation– anion interactions in the crystal
• The higher the coordination number, the more stable the solid
– lowers the potential energy of the solid
• The coordination number depends on the relative sizes of the cations and anions that maintains charge balance
– generally, anions are larger than cations
– the number of anions that can surround the cation is limited by the size of the cation
– the closer in size the ions are, the higher the coordination number is
Ionic Crystals
CsCl coordination number = 8
Cs + = 167 pm
Cl
─
= 181 pm
NaCl coordination number = 6
Na + = 97 pm
Cl
─
= 181 pm
Octahedral
Hole
Lattice Holes
Tetrahedral
Hole
Simple Cubic
Hole
Lattice Holes
• In hexagonal closest-packed or cubic closest- packed lattices there are eight tetrahedral holes and four octahedral holes per unit cell
• In a simple cubic lattice there is one cubic hole per unit cell
• Number and type of holes occupied determines formula (empirical) of the salt
= Octahedral
= Tetrahedral
Cesium Chloride Structures
• Coordination number = 8
• ⅛ of each Cl ─ (184 pm) inside the unit cell
• Whole Cs + (167 pm) inside the unit cell
– cubic hole = hole in simple cubic arrangement of Cl ─ ions
• Cs:Cl = 1: (8 x
⅛), therefore the formula is CsCl
Rock Salt Structures
• Coordination number = 6
• Cl ─ ions (181 pm) in a face-centered cubic arrangement
– ⅛ of each corner Cl ─ inside the unit cell
– ½ of each face Cl ─ inside the unit cell
• Na + (97 pm) in holes between Cl ─
– octahedral holes
– 1 in center of unit cell
– 1 whole particle in every octahedral hole
– ¼ of each edge Na + inside the unit cell
• Na:Cl = (¼ x
12) + 1: (⅛ x
8) + (½ x
6) = 4:4 =
1:1,
• Therefore the formula is NaCl
Zinc Blende Structures
• Coordination number = 4
• S 2─ ions (184 pm) in a face-centered cubic arrangement
– ⅛ of each corner S 2─ inside the unit cell
– ½ of each face S 2─ inside the unit cell
• Each Zn 2+ (74 pm) in holes between S 2─
– tetrahedral holes
– 1 whole particle in ½ the holes
• Zn:S = (4 x
1) : (⅛ x
8) + (½ x
6) = 4:4 =
1:1,
• Therefore the formula is ZnS
Fluorite Structures
• Coordination number = 4
• Ca 2+ ions (99 pm) in a face-centered cubic arrangement
– ⅛ of each corner Ca 2+ inside the unit cell
– ½ of each face Ca 2+ inside the unit cell
• Each F ─ (133 pm) in holes between Ca 2+
– tetrahedral holes
– 1 whole particle in all the holes
• Ca:F = (⅛ x
8) + (½ x
6): (8 x
1) = 4:8 =
1:2,
• Therefore the formula is CaF
2
– fluorite structure common for 1:2 ratio
• Usually get the antifluorite structure when the cation:anion ratio is 2:1
– the anions occupy the lattice sites and the cations occupy the tetrahedral holes
Practice – Gallium arsenide crystallizes in a cubic closest-packed array of arsenide ions with gallium ions in ½ the tetrahedral holes. What is the ratio of gallium ions to arsenide ions in the structure and the empirical formula of the compound?
As = cpp = 4 atoms per unit cell
Ga = ½ (8 tetrahedral holes per unit cell)
Ga = 4 atoms per unit cell
Ga:As = 4 atoms :4 atoms per unit cell = 1:1
The formula is GaAs
Nonbonding Atomic Solids
• Noble gases in solid form
• Solid held together by weak dispersion forces
– very low melting
• Tend to arrange atoms in closest-packed structure
– either hexagonal cp or cubic cp
– maximizes attractive forces and minimizes energy
Metallic Atomic Solids
• Solid held together by metallic bonds
– strength varies with sizes and charges of cations
• coulombic attractions
• Melting point varies
• Mostly closest-packed arrangements of the lattice points
– cations
Metallic Structure
Metallic Bonding
• Metal atoms release their valence electrons
• Metal cation “ islands ” fixed in a “ sea ” of mobile electrons
+ e -
+ e -
+
+
+ e -
+ e -
+ +
+
+ e e -
+
+
+ + + e -
+ e e
+ e -
-
+
+ e e -
+ e -
+ e -
+ + e -
+ + e -
+ +
Network Covalent Solids
• Atoms attached to their nearest neighbors by covalent bonds
• Because of the directionality of the covalent bonds, these do not tend to form closest-packed arrangements in the crystal
• Because of the strength of the covalent bonds, these have very high melting points
– generally > 1000 °C
• Dimensionality of the network affects other physical properties
The Diamond Structure: a 3-Dimensional Network
• The carbon atoms in a diamond each have four covalent bonds to surrounding atoms
– sp 3
– tetrahedral geometry
• This effectively makes each crystal one giant molecule held together by covalent bonds
– you can follow a path of covalent bonds from any atom to every other atom
Properties of Diamond
• Very high melting point, ~3800 °C
– need to overcome some covalent bonds
• Very rigid
– due to the directionality of the covalent bonds
• Very hard
– due to the strong covalent bonds holding the atoms in position
– used as abrasives
• Electrical insulator
• Thermal conductor
– best known
• Chemically very nonreactive
The Graphite Structure: a 2-Dimensional Network
• In graphite, the carbon atoms in a sheet are covalently bonded together
– forming six-member flat rings fused together
• similar to benzene
• bond length = 142 pm
– sp 2
• each C has three sigma and one pi bond
– trigonal-planar geometry
– each sheet a giant molecule
• The sheets are then stacked and held together by dispersion forces
– sheets are 341 pm apart
Properties of Graphite
• Hexagonal crystals
• High melting point, ~3800 °C
– need to overcome some covalent bonding
• Slippery feel
– because there are only dispersion forces holding the sheets together, they can slide past each other
• glide planes
– lubricants
• Electrical conductor
– parallel to sheets
• Thermal insulator
• Chemically very nonreactive
Silicates
• ~90% of Earth ’ s crust
• Extended arrays of Si
O
– sometimes with Al substituted for Si – aluminosilicates
• Glass is the amorphous form
Quartz
• SiO
2 in pure form
– impurities add color
• 3-dimensional array of Si covalently bonded to 4 O
– tetrahedral
• Melts at ~1600 °C
• Very hard
Micas
• There are various kinds of mica that have slightly different compositions – but are all of the general form X
2
Y
4-6
Z
8
O
20
(OH,F)
4
– X is K, Na, or Ca or less commonly Ba, Rb, or Cs
– Y is Al, Mg, or Fe or less commonly Mn, Cr, Ti, Li, etc.
– Z is chiefly Si or Al but also may include Fe 3+ or Ti
• Minerals that are mainly 2-dimensional arrays of Si bonded to O
– hexagonal arrangement of atoms
• Sheets
• Chemically stable
• Thermal and electrical insulator
Practice – Pick the solid in each pair with the highest melting point a) KCl ionic a) KCl
SCl
2 molecular
SCl
2 b) C(s, graphite) cov. network S
8 molecular b) C(s, graphite) c) Kr atomic K metallic
Kr
2 ionic
K
SiO
2
(s, quartz) cov. network d) SrCl
2
SiO
2
(s, quartz)
Band Theory
• The structures of metals and covalent network solids result in every atom ’ s orbitals being shared by the entire structure
• For large numbers of atoms, this results in a large number of molecular orbitals that have approximately the same energy; we call this an energy band
Band Theory
• When two atomic orbitals combine they produce both a bonding and an antibonding molecular orbital
• When many atomic orbitals combine they produce a band of bonding molecular orbitals and a band of antibonding molecular orbitals
• The band of bonding molecular orbitals is called the valence band
• The band of antibonding molecular orbitals is called the conduction band
Molecular Orbitals of Polylithium
Band Gap
• At absolute zero, all the electrons will occupy the valence band
• As the temperature rises, some of the electrons may acquire enough energy to jump to the conduction band
• The difference in energy between the valence band and conduction band is called the band gap
– the larger the band gap, the fewer electrons there are with enough energy to make the jump
Types of Band Gaps and
Conductivity
Band Gap and Conductivity
• The more electrons at any one time that a substance has in the conduction band, the better conductor of electricity it is
• If the band gap is ~0, then the electrons will be almost as likely to be in the conduction band as the valence band and the material will be a conductor
– metals
– the conductivity of a metal decreases with temperature
• If the band gap is small, then a significant number of the electrons will be in the conduction band at normal temperatures and the material will be a semiconductor
– graphite
– the conductivity of a semiconductor increases with temperature
• If the band gap is large, then effectively no electrons will be in the conduction band at normal temperatures and the material will be an insulator
Doping Semiconductors
• Doping is adding impurities to the semiconductor ’ s crystal to increase its conductivity
• Goal is to increase the number of electrons in the conduction band
• n-type semiconductors do not have enough electrons themselves to add to the conduction band, so they are doped by adding electron-rich impurities
• p-type semiconductors are doped with an electrondeficient impurity, resulting in electron “ holes ” in the valence band. Electrons can jump between these holes in the valence band, allowing conduction of electricity.
Diodes
• When a p-type semiconductor adjoins an n-type semiconductor, the result is an p-n junction
• Electricity can flow across the p-n junction in only one direction – this is called a diode
• This also allows the accumulation of electrical energy – called an amplifier