Gram positive bacilli
advertisement

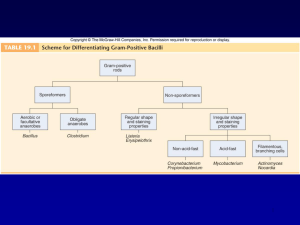
Gram positive bacilli Bacillus spp. Corynebacterium diphteria Erysiphelothrix Listeria spp. Gram positive aerobic bacilli (2 hours): Learning Objectives • 1. Defines ‘’Gram positive aerobic bacilli’’ • 1.1 Lists Gram positive aerobic bacilli in normal flora. • 1.2. Lists pathogenic Gram positive aerobic bacilli for human. • 1.3. Lists virulance factors, defines tissue damage mechanisms. 2. Lists the clinical tables related with Gram positive aerobic bacilli and defines pathogenetic mechanisms. • • 2.1. Defines the clinical importance of Gram positive aerobic bacilli (Bacillus spp., Corynebacterium diphteria,Erysiphelothrix spp, Listeria spp.) • 2.2. Lists the classical culture methods, microscopy and additional diagnostic methods (Schick test, ELEK test) • 2.3. Lists the bioterrorism Gram positive aerobic bacilli agents. Bacillus spp • After coagulase-negative staphylococci and diphtheroids, members of the genus Bacillus are the third most common skin contaminant found in clinical specimens. • On Gram stain they are large, wide, Gram-positive rods, often occurring singly or in pairs, which can produce endospores. • They may be confused with clostridia on direct stains from specimens. Bacillus spp-II • Usually they are readily distinguished from lactobacilli which tend to occur as long, narrow Gram-positive rods which often chain. Also, Bacillus spp. are catalase-positive, while lactobacilli are not • Colony morphology of Bacillus spp. is highly variable, often growing as large to very large gray-white colonies that may be dry in appearance, or which may produce a contiguous mat of wet, blistery colonies. Bacillus spp-III • a genus of Gram-positive bacilli which are commonly found in nature – soil, – water, – airborne dust. • members of natural flora in the human intestines. • Most species of Bacillus are harmless saprophytes, • two species are considered medically significant: – B.anthracis – B. cereus. B. anthracis • causes anthrax in cows, sheep, and sometimes humans. • Anthrax is transmitted to humans via – direct contact with animal products – inhalation of endospores. – ingestion • Sources of infection are usually industrial or agricultural and the infection is classified as one of three types: CUTANEOUS INFECTION (95% of human cases) INHALATION ANTHRAX (rare) : Bioterrorism agent GASTROINTESTINAL ANTHRAX (very rare!) Diagnosis Microscopy: • Gram positive centrally located spore forming bacilli • In tissue imprints – Gram positive capsulated chain forming bacilli Culture: Blood agar Bacillus produces large, spreading, gray-white colonies with irregular margins: Medusa head A unique characteristic of this bacterium is its ability to produce endospores when environmental conditions are stressful. The only other known spore-producing bacterium is Clostridium Diagnosis-II • Typical skin lesion: Dark centered necrotic lesion • Microscopy from the skin lesion: (Specimen should be taken at the margin of healthy and diseased tissue) – Under the microscope, B. anthracis cells appear to have square ends and seem to be attached by a joint to other cells. – The spores are best observed when the bacterium is cultured on artificial media. • • Culture Blood agar: Medusa head-like colonies • LABORATORY INDICATIONS: Nonhemolytic (sheep blood agar) Non-motile Gel hydrolysis Catalase + B. cereus • Unlike B. anthracis, B.cereus is a motile bacterium • cause toxin-mediated food poisoning. • It is known to inhabit many kinds of food – Rice – stew, – cereal, – milk . • The two toxins released by the vegetative form of the bacilli – vomiting – diarrhea, (symptoms similar to those of Staphylococcus food poisoning). • Because toxin production usually takes place after the infected foods are cooked, proper cold storage of food is recommended immediately after preparation. Bacillus subtilis grown in air on sheep blood trypticase soy agar Bacillus cereus Bacillus anthracis Bacillus anthracis Evolution of an anthrax eschar in a 4-year-old boy. (A&B) the lesion when first seen (day 0). Note the arm swollen from the characteristic edema. (C) Day 6 (D) Day 10. (E) Day 15. Although penicillin treatment was begun immediately and the lesion was sterile by about 24 hours, it continued to evolve and resolve as seen The pathogenicity of B anthracis depends on two virulence factors: •a poly-y-D-glutamic acid polypeptide capsule, which protects it from phagocytosis by the defensive phagocytes of the host, •a toxin produced in the log phase of growth. This toxin consists of three proteins: •protective antigen (PA) (82. 7 kDa), •lethal factor (LF) (90.2 kDa), •edema factor (EF) (88.9 kDa). Colonies of B anthracis on a blood agar plate. Note the characteristic tackiness of colonies that allow them to be teased upright with a loop (foreground) and the characteristic tailing seen in the background (arrows). Corynebacterium diphtheriae • aerobic • extracellular • rods; club-shaped, has granules “V”, “L”, or “Chinese Letter” colonies = Dark gray or black on potassium tellurite medium C. diphtheriae-II • no motility • no capsule & glycocalyx • exotoxins = diphtheria toxin – An ADP-Ribosyltransferase • Peptide B binds to host cells to transport peptide A inside • Peptide A has the enzymatic activity • attaches ADP ribose and prevents ribosome movement along mRNA • blocks host EF2 (a protein synthesis elongation factor tRNA translocase) • blocks protein synthesis • can kill host’s NK cells (natural killer) • coded on viral DNA which gets integrated into the bacteria by lysogeny – symptoms: pseudomembrane formation in the throat; exudate forms a tough gray membrane which can lead to stridor (high pitched respiratory sound), respiratory distress, cyanosis, lymphadenopathy – can be fatal pseudomembrane • intoxication consequences of diphtheria toxin – cardiac toxicity - occurs weeks after initial infection – myocarditis, arrhythmias - Can be fatal – neurologic toxicity - occurs only following severe infection - Early (first few days) – paralysis of soft palate and pharynx • later (months later) - peripheral motor neuropathy • diagnosis must be made fast, and is based solely on symptoms • skin infections are rare – Infects an open wound, mostly in persons with poor hygiene - tropics - presents with gray membrane on non-healing wound • Schick Skin Test (rarely performed) – Positive sign is if there is no reaction to toxin when injected intradermally - means that patient is immune • Humans are the only reservoir for C. diphteria. Upper respiratory tract infections and skin lesions • Horizontal transmission occurs via respiratory droplets virulance factors • Diphtheria exotoxin • Storage granules (metachoromatic bodies) – Contain phosphate polymers for highenergy reserve – Stain with metachromatic dye : methylene blue • Growth on Loeffler medium • Metachromatic staining of storage granules • Growth on tellurite medium - black colonies In vitro toxigenicity test Elek Test • In a strip of sterile filter paper antitoxin is impregnated • A heavy inoculum is streaked on a agar surface • Antitoxinated paper is placed on the surface of the agar medium at right angles to the inocula. • Allowed to incubate for 24 hours. • If the organisim is toxigenic a visible line of Ag-Ab precipitate will form Treatment • Penicillin G to kill the organism • Erythromycin in penicillin allergy • Diphtheria antitoxin (horse or humanderived antibodies to the toxin) must be administered immediately VACCINE & TOXOID • Toxoid - inactive toxin is given as part of the DPT vaccine ERYSIPELOTHRIX • E. rhusiopathiae, the only species of this genus, • been found in many farm animals such as pigs, horses, and turkeys • is better known as a veterinary pathogen than as a human pathogen. When cultured on blood agar or some other nutrient medium, Erysipelothrix forms notably large colonies. • Occasionaly, it can infect a human host and cause an inflammatory skin disease, Erysipeloid. • Treatment usually consists of penicillin G, ampicillin, or cephalothin. • LABORATORY INDICATIONS: Catalase - Non-motile TSI : H2S + LISTERIA • Gram-positive rod which is not capable of forming endospores. • several species exist, but two species are important for human pathogenic significance: • L. monocytogenes • L. ivanovii. Listeria monocytogenes • normal inhabitant of the gastrointestinal tract and of animal feces • Found responsible in several food poisoning epidemics. – led to a 1986 outbreak in Massachusetts hospital patients. – Those infected suffered from vomiting, nausea, and diarrhea. – the hospital patients contracted the microbe from the infected hospital food and were at high risk of infection. • High risk group: – newborns, – pregnant women and their fetuses, – the elderly, – immune compromized Clinical findings: • • • septicemia meningitis listeriosis: is an inflammation of the brain. LABORATORY INDICATIONS: Catalase + Motile at room temperature • Growth at 4 degrees Celsius • Bile esculin hydrolysis • Beta-hemolysis Treatment: • Antibiotics are recommended for treatment of infection • most strains of Listeria are sensitive to ampicillin and gentamicin