Homework - Teach.Chem
advertisement

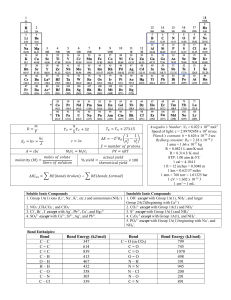
Name: ________________________ Hour: ____ Date: ___________ AP Chemistry: 8HW Circle the compound in each group that has the higher lattice energy. 1A. Group 1: LiCl KCl 1B. Group 2: MgBr2 MgS Group 3: NaF NaCl Group 4: Ni(OH)2 Ni(OH)3 Write the shorthand electron configuration for each of the following. 2A. Mg2+ 2B. N3– K+ Cl– Fe3+ Mo4+ Classify each (“ionic only,” “covalent only,” or “ionic and covalent”) according to the bonds they contain. 3A. (NH4)2O K2S P4O10 3B. RbCl Ba3(PO4)2 Arrange the species in each set in order of increasing size. 4A. Cu Cu+ Cu2+ Ni2+ Pd2+ Pt2+ 4B. O O– O2– La3+ Eu3+ Gd3+ Yb3+ Use the Periodic Table to predict the most polar bond in each of the following groups. Circle your prediction. 5A. Group 1: C–F Si–F Group 2: P–Cl S–Cl Ge–F 5B. Group 3: S–F S–Cl S–Br Group 4: Ti–Cl Si–Cl Ge–Cl 6. Rank the following bonds in order of increasing ionic character: N–O, Mg–O, C–F, Cl–Cl, K–Br 7. Indicate the bond polarity IN TWO WAYS for each of the following. C–O O–P Cl–Te Se–S Draw a correct Lewis structure for each of the following. 8A. HCN PH3 CHCl3 NH4+ 8B. CO2 O2 H2CO SeF2 Assign formal charges to each atom in the following species. 9A. POCl3 9B. ClO4– Draw Lewis structures for each of the following. 10A. NO2– 10B. CO32– 11B. ICl4– Draw Lewis structures for each of the following. 11A. PCl5 SF4 AsF6– Use tabulated bond energy values to estimate the reaction enthalpy for the following gas-phase reactions. 12A. H2 + Cl2 2 HCl 13. Arrange the following species with respect to the carbon-oxygen bond length, longest to shortest. 12B. N2 + 3 H2 2 NH3 CO CO2 CO32– CH3OH